Clean-in-place (CIP) processes are essential to industries such as food and beverage, personal care products, and any other batch processes where residual contamination can negatively impact product quality.
Critical factors in clean-in-place management
Time
Time spent conducting CIP cycles is time that is not spent on production, so CIP cycles should be as short as possible while still being effective.
Chemicals
Under-dosing of cleaning chemicals increases the risk of contamination and biofilm formation and will also necessitate additional chemical treatments to mitigate these more serious issues later. Conversely, over-dosing of chemicals also carries a significant cost and can upset downstream wastewater treatment processes.
Water
Depending on the size of vessels and lines that require cleaning, water requirements can be excessive. These costs can be compounded by the amount of energy and chemical needed when water usage increases.
Energy
The costs associated with pumping as well as heating of water and cleaning agents is directly tied to the amount of water and chemical required. Considering that electricity costs are often among the largest expenses in operating industrial processes, these costs can escalate quickly if water and chemical usage is not optimized.
How can the effectiveness of a CIP cycle be quickly verified?
A holistic water quality monitoring toolbox including rapid microbiological monitoring enables all aspects of CIP cycles as well as the production process as a whole to be optimized.
ATP monitoring has been used for decades in food processing to quickly assess surface cleanliness. While this technology still serves an important role, 2nd Generation ATP focuses on analysis of water and other aqueous samples. In doing so, it greatly expands the applications for this rapid method and enhances QA/QC programs to a significant degree.
Example 1: Food processing QA/QC
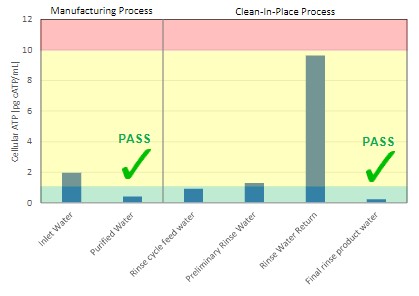
Figure 1 illustrates how the water cycle within a food processing facility can be quickly characterized in terms of risk. In addition to screening municipal inlet water, the cleanliness of purified feed water can be verified in minutes so that contamination incidents can be detected at the earliest point. Considering that make-up water is often the fastest and most common route of contamination, rapid surveillance of this critical control point is of utmost importance.
Water quality can also be closely monitored to ensure clean water for CIP cycles and that adequate performance is achieved. High ATP levels in final rinse water is indicative of incomplete cleaning, meaning that process should continue until a sufficiently low ATP result is returned. Similarly, being able to immediately verify clean rinse water enables immediate termination of the CIP process, thereby avoiding excessively long cleaning cycles and water wastage.
Example 2: Beer Brewing CIP
As is the case in food processing, CIP systems are used extensively in breweries to sufficiently clean fermenters after beer brewing runs. Contaminated beer impacts the quality of the beer (i.e., flavour, aromas, or haziness), and in the worst case, can result in batch spoilage.
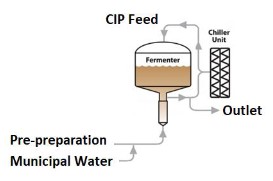
Following each batch, effective cleaning of the fermenter is essential prior to the next batch. If cleaning is not adequate, the following fermentation run could be inhibited due to the presence of bacteria. While bacteria can be detected using selective bacterial counts or microscopic analyses, it is too late to apply corrective action once results are known. Rapid microbiological analyses using a rapid method such as 2nd Generation ATP monitoring provide a potential solution to this challenge.
Figure 3 shows the results of a CIP process around a fermentation run. The feedwater ATP tests prior to entering the chiller system indicate water with very low bioburden levels, but the outlet of the chiller showed far greater values.
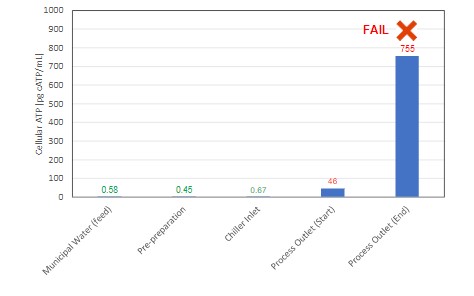
This is indicative of biofilm/residue accumulation in the chiller, so the goal of the CIP cycle should be to reduce this to lower levels. However, after a rinsing cycle, the ATP concentration at the chiller outlet was significantly higher than when the process began. This clearly indicates that the cycle must continue to adequately purge the accumulated material from the system and reduce the risk of contamination to a safe level.
Summary
2nd Generation ATP monitoring represents a major leap forward in rapid microbiological field testing.
In general, the addition of this technology facilitates the following opportunities for CIP optimization:
- Reveal contaminated product at the earliest possible point.
- Optimize inspections and cleaning cycles to close pathways of contamination and minimize downtime between batches, thereby maximizing profitability.
- Streamline equipment maintenance and servicing (e.g., filters, purification systems).
LuminUltra’s value proposition traditionally states that our technology helps “save time, save money, and save water.” However, the integration of 2nd generation ATP monitoring to QA/QC programs in the food & beverage industry can have direct impact of profitability.
Contact us today to learn more about how we can help!