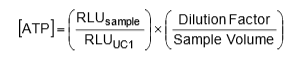One of the major benefits of LuminUltra’s 2nd generation ATP monitoring over existing technology (i.e. ATP pens) is the normalization of enzyme activity. At the microbiological level, knowing how active the enzyme is at the time of testing offers significant benefits for both data collection and analysis. Given this, LuminUltra’s 2nd Generation ATP test kits come equipped with UltraCheck 1, an ATP standard solution with a concentration of 1 ng ATP/mL for calibration of the Luminase enzyme. The calibration accounts for changes in enzyme activity and allows ATP to be converted from Relative Light Units (RLU) to a mass-based concentration (i.e. pg/mL).
As noted above, the output of an ATP test is light, which is measured by a luminometer and reported in RLU’s. In a perfect world, the enzyme performance would be completely independent of external conditions and give consistent results. Unfortunately like most enzymes, Luminase is subject to degradation over time, resulting in a gradual decay of enzymatic activity. Therefore, by reacting Luminase with a known concentration of ATP with UltraCheck 1, we can get a direct indication of the current enzymatic activity and normalize the sample RLU reading. Thus, actual readings from samples gathered in the field can be compared on a common scale.
Temperature is a significant contributor to enzymatic degradation and when the ambient temperature passes the recommended storage threshold of 30°C (86°F), enzymatic activity undergoes an exponential decay which can underestimate the microbial count. In fact, it has been shown that exposure to 45-50°C (113-122°F) temperatures is enough to kill all enzymatic activity within an hour. To address this, a quick calibration step will account for any diminished enzyme activity due to high temperatures out in the field.
Using the data provided by the calibration, the user can determine the viability of the enzyme activity. Only enzyme that passes the RLU threshold provided by the LuminUltra test kit instructions should be used for testing. In the case it doesn’t, a new vial of Luminase must be rehydrated to ensure maximum sensitivity in testing.
On another important note, since the calibration is giving the user a light measurement (RLU) for a known concentration of ATP, the user can convert results from RLU’s to an ATP concentration. The formula for this conversion can be applied across all LuminUltra test kits and is provided below:
For convenience of the user, LuminUltra has already integrated this equation into its Cloud Software with the appropriate dilution factors and sample volumes depending on the application. From this, an operator can get their results in a standardized mass-based form that allows for comparisons between past, present and future data.
Another benefit of the conversion is LuminUltra’s interpretation guidelines in each test kit instruction package. These guidelines provide a solid starting point for those just rolling out their ATP monitoring program. An example of this can be seen below for LuminUltra’s QGA cATP (cellular ATP) test kit guidelines:
| Application: | Good Control (pg cATP/mL) | Preventive Action (pg cATP/mL) | Corrective Action (pg cATP/mL) |
|---|---|---|---|
| High-Purity Water | < 0.1 | 0.1 to 1.0 | > 1.0 |
| Portable & Sanitary Water | < 1 | 1 to 10 | > 10 |
| Raw Make-up Water (Fresh, Brackish, Salt, Reclaimed) | < 10 | 10 to 100 | > 100 |
| Cooling & Process Water Oxidizing Biocides* | < 10 | 10 to 100 | > 100 |
| Cooling & Process Water Non-Oxidizing Biocides or Non-Chemical Treatment* | < 100 | 100 to 1,000 | > 1,000 |
By using the calibrated enzyme activity, in alliance with the interpretation guidelines, an operator can get an indication on whether the system is in good control or not.
Given the described importance behind ATP standard calibrations, LuminUltra recommends one is performed each day or for each set of samples analyzed at the same time. As well, calibration is a must anytime a new bottle of Luminase is being rehydrated for use.
To see more added benefits of LuminUltra’s 2nd Generation ATP technology over 1st Generation methods, sign up for the full technical whitepaper. Additionally, for a visual breakdown of the potential savings in switching over from traditional culture-based methods, check out this blog post.
Further reading:
[Slide Deck] Extracting Value from 2nd Generation ATP® Monitoring