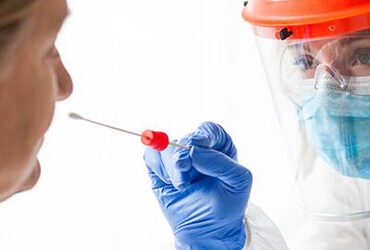
Voluntary passenger and employee testing onsite provides accurate results in under two hours
TORONTO, ON — The Greater Toronto Airport Authority announced this morning that starting March 1 it will use LuminUltra’s game-changing rapid, portable COVID-19 qPCR testing solution on-site at Pearson International Airport, as part of a federally funded research study. The biotechnology front-runner’s GeneCount® COVID-19 RT-qPCR Assay Kit is authorized by Health Canada for sale and distribution.
PCR is the gold-standard for diagnosing a COVID-19 infection. In most cases, samples are collected at mobile testing sites and shipped to centralized laboratories for testing. This process can lead to delays and longer wait times for results — in some cases days or even weeks. LuminUltra’s portable, point-of-need devices can be used on-site, providing results in under two hours.
LuminUltra’s GeneCount® COVID-19 PCR solutions are being used on-site at the Greater Toronto Airports Authority (GTAA) as part of a COVID-19 testing research program funded by the Government of Canada. The program is designed to explore the efficacy of antigen testing in comparison to the gold-standard PCR tests. Participants in the study will be provided with the results of their PCR test while onsite at the airport.
“LuminUltra is proud to provide our made-in-Canada COVID-19 testing solution to the GTAA,” says Pat Whalen, Chairman and CEO of LuminUltra. “Our rapid, on-site qPCR technology provides real-time insight into the health of staff and passengers, helping to prevent further spread and acting as the gold-standard comparison for the study. Continued investigation into how to best serve our workplaces is essential as we continue to navigate this pandemic.”
LuminUltra is also working with other customers to implement its solutions in facilities such as hospitals and mobile labs that can travel to different communities, as needed if, for instance, an outbreak occurs and supplementary testing capacity is required.
“We’ve worked to develop a range of flexible solutions that can meet a variety of needs,” says Whalen. “From small, portable devices that can be moved to different locations of a hospital to large scale, high-throughput machines that can be used in a mobile lab that can drive to locations of need, our technology brings more gold-standard PCR testing to where it is needed and greatly reduces time between test and results, getting employees back to work sooner and making communities safer for all.”
LuminUltra’s GeneCount® COVID-19 testing solutions comprise multiple, flexible components that together make up a complete laboratory-grade point-of-need solution, including: sample collection kits; isolation reagents; the COVID-19 assay, which works for all known variants; and a range of qPCR testing devices and software for simple interpretation of COVID-19 test results with industry-leading accuracy and sensitivity. The GeneCount platform includes instruments tailored to two specific needs:
- Portable, point-of-need devices built into a carrying case that enable up to 16 tests per run to be performed where they are needed; and
- High capacity, automated devices that enable up to 96 tests per run ideal for both mobile and traditional brick-and-mortar laboratories.
Since March of last year, LuminUltra has recognized the important role of testing in the fight against COVID-19 and has focused on increasing capacity to meet the testing needs of all of Canada. Underscoring its reliable supply chain, LuminUltra responded promptly to the Government of Canada’s testing needs and has been a key supplier of the isolation reagents for the national testing program since April 2020, keeping up with the demand of national testing needs by providing 500,000 tests a week.
-30-
About LuminUltra
Founded in 1995, LuminUltra is a biological diagnostic testing company headquartered in Canada with operations in six countries. It is widely recognized globally as a leader in developing tests and reagents for environmental, industrial, and diagnostic monitoring and is a key supplier of COVID-19 clinical testing reagents to the Government of Canada. Customers in over 80 countries trust LuminUltra’s technology, production reliability and history of customer service excellence to deliver their essential services in a safe-state. At the same time, LuminUltra fosters a culture of innovation and agility and is on an accelerated growth path, acquiring multiple companies in recent years and forming a partnership with the specialized private equity firm XPV Water Partners. Additional information can be found here.
Media contact
media@luminultra.com
LuminUltra’s complete COVID-19 testing solutions give governments and businesses the tools they need to deliver a high volume of the most accurate COVID-19 test results possible in under two hours. Learn how these products can be tailored to your specific needs.